In the reaction of nitrogen gas with oxygen gas to produce nitrogen oxide, what is the effect of adding more oxygen gas to the initial reaction mixture? The reaction is shown below. 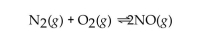
A) The equilibrium is not affected.
B) The equilibrium shifts to produce more 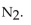
C) The temperature of the reaction mixture is raised.
D) The equilibrium shifts to produce more NO.
E) Extra catalyst is required to reach equilibrium.
Correct Answer:
Verified
Q24: In the reaction of carbon dioxide with
Q25: In an exothermic reaction, heat can be
Q26: For the following reaction, the equilibrium constant
Q27: For the following reaction, the equilibrium constant
Q28: When you open a bottle of a
Q30: Carbon monoxide binds to hemoglobin 140 times
Q31: Treatment of carbon monoxide poisoning can be
Q32: In the following gas phase reaction, what
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents