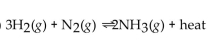 For the reaction at equilibrium, if the volume is increased, will the equilibrium shift in the direction of reactants, products, or stay the same?
For the reaction at equilibrium, if the volume is increased, will the equilibrium shift in the direction of reactants, products, or stay the same?
A) reactants
B) products
C) stay the same
Correct Answer:
Verified
Q42: The rule or principle that describes the
Q43: Write the equilibrium expression for the reaction
Q44: Q45: What is the correct form of the Q46: For the following reaction, the equilibrium constant Q47: An equilibrium constant with a value greater Q48: The equilibrium between hemoglobin and oxyhemoglobin in Q49: The _ is the energy difference between Q51: A mixture at equilibrium that contains less Q52: ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents