Consider the following reaction. 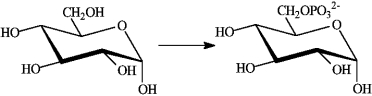 Which of the following describes this reaction?
Which of the following describes this reaction?
A) is so strongly exergonic that it does not require a catalyst
B) is an exergonic reaction not coupled to any other reaction
C) is an endergonic reaction that takes place because it is coupled to the exergonic hydrolysis of ATP
D) is an exergonic reaction that is coupled to the endergonic hydrolysis of ATP
Correct Answer:
Verified
Q12: Instructions: Consider the reaction below to answer
Q13: Which group of small molecules best fit
Q14: What reaction does glutamate dehydrogenase (GDH)
Q15: What product would be obtained from enzymatic
Q16: The formation of amino acids from proteins
A)
Q18: Which group of small molecules best fit
Q19: Which group of small molecules best fit
Q20: What amino acid is the following
Q21: The source of oxygen for
Q40: When acetyl-CoA reacts with oxaloacetate to form
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents