Treatment of tert-butyl alcohol with hydrogen chloride yields a mixture of tert-butyl chloride and 2-methylpropene. 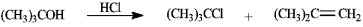 a)After chromatographic separation, how would you use 1H NMR to help you decide which was which?
a)After chromatographic separation, how would you use 1H NMR to help you decide which was which?
b)How would the 13C NMR for the two compounds differ?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q48: A compound with the molecular formula C6H4ClBr
Q49: Instructions: For each of the compounds below
Q50: Instructions: For each of the compounds below
Q51: Instructions: Predict the splitting patterns you would
Q52: Instructions: Answer the following question(s) for the
Q54: Instructions: Answer the following question(s) for the
Q55: Instructions: Predict the splitting patterns you would
Q56: A compound with the molecular formula C5H12O
Q57: Instructions: For each of the compounds below
Q58: Which structure of molecular formula C4H8Cl2 fits
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents