The wave functions of some molecules are a combination of wave functions with different values of the orbital quantum number .The wave function of PF5 combines s, p and d states in an sp3d hybrid orbital.We would expect such an overlap of wave functions in individual molecules to represent: 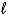
A) ionic bonding.
B) metallic bonding.
C) covalent bonding.
D) Van der Waal's bonding.
E) hydrogen bonding.
Correct Answer:
Verified
Q21: The Fermi energy corresponds to
A) the maximum
Q22: The Fermi temperature of copper is 80
Q28: In comparing vibrational and rotational levels in
Q30: Because HF, hydrogen fluoride, is a covalent
Q31: When calculating the rotational kinetic energy of
Q31: An energy band in a solid consists
Q35: When a voltage
Q36: The Fermi temperature is:
A)a characteristic temperature
Q38: The energy gap for germanium is 0.670
Q39: The energy of a molecule can normally
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents