One of the main problems with the Bohr model of the hydrogen atom when compared with the results of the methods of quantum mechanics used to describe atoms, was that the Bohr model predicted:
A) the ground state angular momentum was L = 1 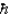
B) the frequency of the radiation emitted when an electron 'jumps' from one allowed orbit to another was hf = Ei - Ef.
C) the potential energy function for the hydrogen atom was given by V(r) = -ke2/r.
D) the energy of the ground state of the hydrogen atom was En =-13.6 eV.
Correct Answer:
Verified
Q1: The probability density for the 1s
Q2: In the subshell of the Li2+ ion
Q2: Suppose Bohr had chosen the potential energy
Q3: How fast is the electron moving in
Q4: A hydrogen atom is in its first
Q6: The allowed values of for the n
Q8: The probability density of a particle at
Q9: For the following allowed transitions, which photon
Q11: An energy of 13.6 eV is needed
Q15: An electron in a hydrogen atom makes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents