If the rms speed of helium atoms is vrms,He at temperature T, what is the rms speed of CO2 at the same temperature?
A) 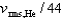
B) 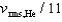
C) 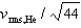
D) 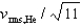
E) 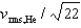
Correct Answer:
Verified
Q21: The temperature of a quantity of an
Q23: Two tanks of gas, one of hydrogen,
Q24: Two tanks of gas, one of hydrogen,
Q25: The specific heat of an ideal gas
Q25: If CP for an ideal gas is
Q27: If the total translational kinetic energy of
Q30: The molar specific heat at constant
Q33: According to kinetic theory, a typical gas
Q67: Air expands adiabatically (no heat in,no
Q69: One mole of helium gas expands
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents