A gas expands as shown in the graph. If the heat taken in during this process is 1.02 × 106 J and 1 atm = 1.01 × 105 N/m2, the change in internal energy of the gas (in J) is how much? 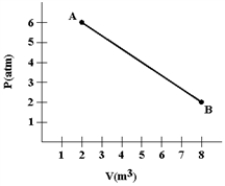
A) −2.42 × 106
B) −1.40 × 106
C) −1.02 × 106
D) 1.02 × 106
E) 1.40 × 106
Correct Answer:
Verified
Q25: Water at room temperature, 20°C, is pumped
Q27: A styrofoam container used as a picnic
Q34: A block of material of mass m
Q42: Duff states that equal masses of all
Q43: We are able to define a mechanical
Q44: Which of the following substances has the
Q45: Beryl states that insulation with the smallest
Q53: A 100-g cube of ice is heated
Q54: In a thermodynamic process, the internal energy
Q56: 100 grams of liquid nitrogen at 77
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents