The average molecular translational kinetic energy of a molecule in an ideal gas is
A) 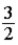 kBT.
kBT.
B) 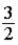 RT.
RT.
C) 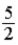 kBT.
kBT.
D) 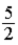 RT.
RT.
E) 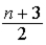 kBT, where n = number of internal degrees of freedom.
kBT, where n = number of internal degrees of freedom.
Correct Answer:
Verified
Q3: An ideal gas is allowed to expand
Q6: A 50-gram sample of dry ice (solid
Q7: A container having a volume of 1.0
Q10: A container having a volume of 1.0
Q11: During the volcanic eruption of Mt. Pelee
Q11: Which statement below is NOT an assumption
Q14: A molecule in a uniform ideal gas
Q21: The temperature of a quantity of an
Q24: Two tanks of gas, one of hydrogen,
Q33: The root mean square speed of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents