Equal volumes of hydrogen and helium gas are at the same pressure. The atomic mass of helium is four times that of hydrogen. If the total mass of both gases is the same, the ratio of the temperature of helium (He) to that of hydrogen (H2) is
A) 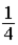 .
.
B) 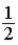 .
.
C) 1.
D) 2.
E) 4.
Correct Answer:
Verified
Q9: Two identical containers, A and B, hold
Q22: Two bodies can be in thermal equilibrium
Q25: A bubble having a diameter of 1.00
Q26: A bowling ball size probe from a
Q26: The mass of a sulfur atom is
Q30: A temperature difference of 9.0 Celsius degrees
Q32: Equal masses of hydrogen and helium gas
Q33: A square plate has an area of
Q35: Angela claims that she wears a cylindrical-shaped
Q46: A constant volume gas thermometer has a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents