In the figure below, a negative ion is surrounded by water molecules. 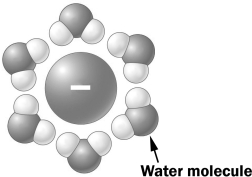
The water molecules are oriented as shown above because the slightly _____________ in the water molecule is (are) attracted to the ion.
A) negative hydrogen atoms
B) positive hydrogen atoms
C) negative oxygen atom
D) positive oxygen atom
Correct Answer:
Verified
Q3: The strongest possible chemical link between two
Q4: An atom of the element with the
Q4: The outer electron shell of a nitrogen
Q7: Ionic bonds
A) result from the sharing of
Q8: Which of the following combinations of atoms
Q8: Individual water molecules are pulled toward each
Q9: The figure below shows two hydrogen atoms.
Q11: The smallest unit of a chemical element
Q12: Oil and water do not mix together
Q37: In the equation 3H₂ + N₂ à
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents