This graph depicts the amount of energy involved over the course of a chemical reaction. 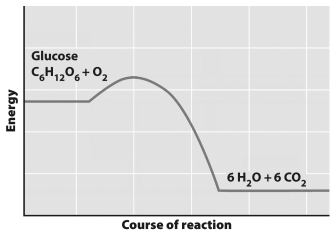
The graph indicates that
A) the products of this reaction have more energy that the substrates.
B) this reaction requires an input of energy to convert glucose and O₂ to H₂O and CO₂.
C) this reaction can only run in one direction (from left to right on the graph) .
D) this reaction only occurs in the presence of an enzyme.
Correct Answer:
Verified
Q4: When humans cut down trees for lumber
Q12: Heat speeds up chemical reactions by
A) causing
Q15: The second law of thermodynamics states that
A)metabolic
Q17: _ reactions use energy to build complex
Q20: The energy required for life processes must
Q20: In the reaction X + Y →
Q23: When ATP breaks down into ADP and
Q28: A molecule of sugar slowly "burns" in
Q36: In the reaction CH₂O + O₂ →
Q38: Which of the following compounds is the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents