The synthesis of ammonia from hydrogen and nitrogen produced the following kinetic data: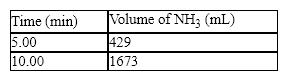 What is the average rate in mL/min for the second five-minute period of the reaction?
What is the average rate in mL/min for the second five-minute period of the reaction?
A) 167 mL/min
B) 85.3 mL/min
C) 249 mL/min
D) 429 mL/min
Correct Answer:
Verified
Q19: The rate constant "k" will vary with
Q20: Which statement best describes the rate of
Q21: Consider the reaction: 2A + B →
Q22: The decomposition of hydrogen peroxide (H2O2) into
Q23: A catalyst elevates the rate of a
Q25: Consider the reaction: 2A + B →
Q26: Consider the reaction: A + 2B →
Q27: During the formation of MgO from the
Q28: Raising the temperature of a reaction elevates
Q29: The synthesis of ammonia from hydrogen and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents