Exhibit 2B Contains information on the pK's of some common buffers. 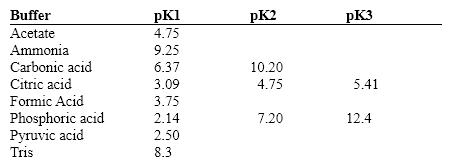 Refer to Exhibit 2B. A carbonate buffer would work well at this pH:
Refer to Exhibit 2B. A carbonate buffer would work well at this pH:
A) 4.0
B) 6.0
C) 8.0
D) 10.0
E) 6.0 and 10.0
Correct Answer:
Verified
Q65: Exhibit 2B Contains information on the pK's
Q66: Which substance would be the best buffer
Q67: Buffering capacity refers to
A) the effectiveness of
Q68: Exhibit 2B Contains information on the pK's
Q69: If the pH of 1 liter of
Q71: Exhibit 2B Contains information on the pK's
Q72: Which of the following is true?
A) The
Q73: A buffer solution
A) is used to control
Q74: Using the Henderson-Hasselbalch equation, calculate the pH
Q75: If the pH of 1 liter of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents