For a general equation , the relation between the free-energy change ( ) for the reaction under any condition and the free energy change under standard conditions ( ) can be written as _____.
A) 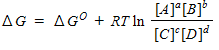
B) 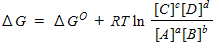
C) 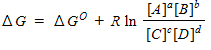
D) 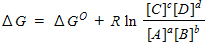
Correct Answer:
Verified
Q55: Which of the following are examples of
Q56: When we say that the efficiency of
Q57: Spontaneous reaction always occurs at a relatively
Q58: The linking of an exergonic reaction to
Q59: In order to initiate many metabolic pathways
Q60: The hydrolysis of ATP can be used
Q61: Explain what happens to carbon atoms in
Q62: Identify the value of if a
Q63: Among the following organophosphates, which compound
Q65: Explain the differences between catabolism and anabolism.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents