If an isotope has a half-life of 4 billion years, then in 4 billion years
A) all of the original amount will have decayed.
B) 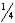 of the original amount will still remain.
of the original amount will still remain.
C) 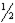 of the original amount will still be present.
of the original amount will still be present.
D) all of the original amount will still be present.
E) none of these
Correct Answer:
Verified
Q28: What is the term used for small
Q29: Carbon-14 dating is most appropriate for dating
A)organic
Q30: Striking a core or flake with a
Q31: _ is a radiometric dating method popular
Q32: Which is NOT true about thermoluminescence dating?
A)It
Q34: Which dating method was used to date
Q35: Jolly's seed-eating hypothesis is
A)considered the best supported
Q36: Which of the following would be best
Q37: Which of these factors is NOT considered
Q38: A stone reduced by flake removal is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents