Figure 2-1 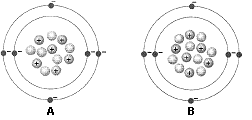 What is the difference between the two atoms in the accompanying figure?
What is the difference between the two atoms in the accompanying figure?
A) Their electrical charge
B) The number of electrons
C) The number of protons
D) The number of neutrons
E) The number of valence shells
Correct Answer:
Verified
Q1: Figure 2-1 Q2: How can we identify a particular element? Q4: The location and/or metabolism of a substance Q5: Which best statement describes an element? Q7: In a water molecule, because oxygen is Q8: What type of bond is formed if Q9: Which substance correctly identifies a reactant in Q10: The chemical behavior of an atom is Q11: The molecular mass of C6H12O6 is 180 Q13: The representation H−O−H is known as a(n):![]()
A)
A) A
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents