Figure 7-1 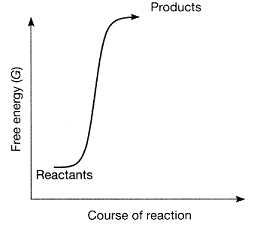 Which statement best describes the plot in the accompanying figure?
Which statement best describes the plot in the accompanying figure?
A) The figure represents an endergonic reaction.
B) The figure represents a spontaneous reaction.
C) The products have more free energy than the reactants.
D) The reactants have more free energy than the products.
E) The reaction is endergonic, and in addition, the products have more free energy than the reactants.
Correct Answer:
Verified
Q1: Consider the following two chemical equations:
A.
Q2: In a reaction in which the rate
Q3: Every type of chemical bond contains a
Q4: Energy that is useable and organized is
Q5: You are out cycling and start to
Q6: What process occurs when complex molecules are
Q7: An organism can exchange matter and energy
Q8: What is the ultimate source of energy
Q9: As you climb a flight of stairs,
Q11: Suppose the free energy of the reactants
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents