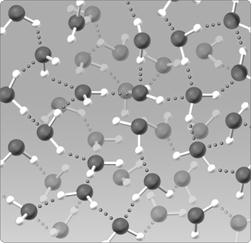
Figure 2.5
The water lattice illustrated in the figure above forms as a result of ____ between water molecules.
A) covalent bonds
B) hydrogen bonds
C) nonpolar interactions
D) ionic bonds
E) van der Walls forces
Correct Answer:
Verified
Q48: Water has an important stabilizing effect on
Q50: Water has an unusually high boiling point
Q52: When water molecules exposed to the air
Q55: A molecule of water in the middle
Q57: Molecules such as H-H and O=O are
Q61: Lemon juice has a pH of 2.0,
Q63: Biological membranes are held together mainly by
Q66: High levels of carbon dioxide in the
Q77: When sugar dissolves in water, water is
Q80: In water, NaOH almost completely separates into
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents