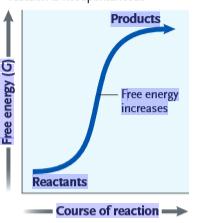
Figure 6.1
What can be inferred from the accompanying graph?
A) This reaction is endergonic, with a positive ΔG.
B) This reaction is endergonic, with a negative ΔG.
C) This reaction is exergonic, with a positive ΔG.
D) This reaction is exergonic, with a negative ΔG.
E) There is not enough information to make a determination.
Correct Answer:
Verified
Q23: Which example would have a negative change
Q26: Which statement is true for exergonic reactions?
A)
Q27: An exergonic reaction will have a _.
A)
Q28: The free energy of ATP hydrolysis is
Q32: When an enzyme-catalyzed reaction reaches equilibrium _.
A)
Q35: We can calculate whether a reaction is
Q36: Eating and digesting a candy bar for
Q39: Which reaction is most likely to have
Q52: If an enzyme is saturated, _.
A) it
Q56: Where does the energy for ATP synthesis
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents