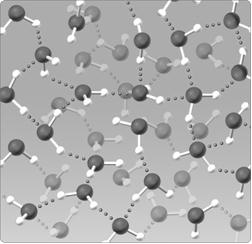
 Figure 2.5
Figure 2.5
The water lattice illustrated in the figure above forms as a result of ____ between water molecules.
A) covalent bonds
B) hydrogen bonds
C) nonpolar interactions
D) ionic bonds
E) van der Walls forces
Correct Answer:
Verified
Q48: Water has an important stabilizing effect on
Q49: Q50: Water has an unusually high boiling point Q51: A molecule of water in the middle Q52: When water molecules exposed to the air Q54: In contrast to ionic bonds, covalent bonds Q55: Multiple hydrogen bonds together stabilize proteins into Q56: The formation and breaking of bonds between Q57: Molecules such as H-H and O=O are Q58: ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents