There are _____ valence electrons available for the Lewis dot structure for 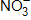 .
.
A) 23
B) 24
C) 27
D) 20
Correct Answer:
Verified
Q25: According to the valence shell electron pair
Q25: Which of the following has the largest
Q26: If the combined radii of each pair
Q26: Which of the following compounds illustrates sp3
Q27: Which of the following molecules has the
Q28: According to the valence shell electron pair
Q28: Which of the following has the largest
Q29: Draw the Lewis dot structure of nitrate.
Q33: The symbol for chloride ion is _.
A)Cl
Q34: Which of the following has the largest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents