Consider the following reaction: 2A + B → C.
A kinetics study on this reaction yielded the following data: 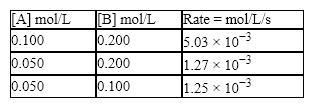 What is the value of the rate constant?
What is the value of the rate constant?
A) 3.20 mol L − 1s
B) 19.8 mol L −1 s
C) 0.508 mol L −1 s
D) 4.60 mol L −1 s
Correct Answer:
Verified
Q6: For a first-order reaction, t1/2 = ln
Q19: For a first order reaction, the plot
Q21: A catalyst increases the rate of a
Q22: The three-way catalytic converters found in automobile
Q23: Consider the following reaction:
A + 2B →
Q25: For a zero-order reaction, the plot of
Q26: Consider the following elementary step: A +
Q28: The synthesis of ammonia from hydrogen and
Q29: The production of nitric oxide is governed
Q39: If the decomposition of reactant A follows
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents