The production of ammonia N2( g ) + 3H2( g ) 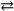 2NH3( g ) represents a(n) _____.
2NH3( g ) represents a(n) _____.
A) homogeneous equilibrium
B) heterogeneous equilibrium
C) instantaneous equilibrium
D) reaction that will not come to an equilibrium
Correct Answer:
Verified
Q2: According to the Brønsted-Lowry theory of acids
Q10: "Insoluble" salts are actually "sparingly" soluble at
Q12: In the context of exothermic reactions in
Q13: If a reaction is in a state
Q13: Products appear in the numerator of the
Q14: The higher the K sp of
Q17: Unlike traditional concrete, engineered cementitious composites (ECC)do
Q18: If NaCl is added to a saturated
Q19: A catalyst will increase the rate of
Q20: The term "strong acid" refers to any
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents