Potassium hydrogen phthalate (KHP) concentrations can be determined through titrating samples of KHP (a monoprotic acid) with bases such as NaOH in the presence of an indicator such as phenolphthalein. The indicator is colorless in an acidic solution and turns pink in an alkaline solution. Thus, we can establish an equilibrium for the phenolphthalein with the following reaction.
HIn + H2O 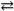 In − + H3O+
In − + H3O+
If the HIn species is "acid color" or colorless for the phenolphthalein, and the In − species is "base color" or pink for this particular indicator, what color will appear in a flask in which a 0.2993 gram sample of KHP is completely neutralized with an excess of NaOH?
A) The flask will be colorless.
B) The flask will be pink.
C) The flask will be white from KCl precipitation.
D) There is insufficient information to solve this problem.
Correct Answer:
Verified
Q21: The conjugate acid of sodium acetate (Na+CH3COO
Q21: At equilibrium, Δ G _.
A)= 0
B)> 0
C)<
Q27: Which of the following is a strong
Q27: The synthesis of the pesticide thionyl chloride
Q28: The conjugate base of ammonium ion is
Q29: Which of the following chemical species is
Q30: Consider the following reaction of a weak
Q31: What change in the reaction direction occurs
Q35: What is the pH of a 0.1055
Q40: What is the molar solubility of AgCl
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents