What is the cell potential (E0) for a galvanic cell formed from the following two half-reactions? Assume that the cell temperature is 45°C and the operating pressure is 0.06 atm. 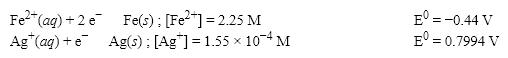
A) 1.03 V
B) 1.12 V
C) 1.24 V
D) 0.36 V
Correct Answer:
Verified
Q24: Balance the following electrochemical reaction in acid:
MnO4
Q35: Which of the following cells is most
Q37: Single use, non-rechargeable batteries are referred to
Q37: The most common fuel cells are based
Q38: What is the cell potential (E
Q41: In the context of the cell notation
Q42: Which of the following represents the Nernst
Q43: An electrolysis cell that deposits copper (from
Q44: In general, a secondary cell has a
Q45: In any galvanic cell, the half-reaction with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents