Calculate the energy released by a nucleus of uranium-235if it splits into an antimony-132nucleus and a niobium-101nucleus according to the equation below. 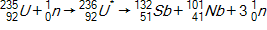 Consider the atomic masses of
Consider the atomic masses of 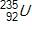 ,
, 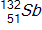 ,
, 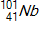 , and
, and 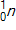 to be 235.044 u, 131.915 u, 100.915 u, and 1.00866 u, respectively.
to be 235.044 u, 131.915 u, 100.915 u, and 1.00866 u, respectively.
A) -1.53 × 10- 11 J
B) -2.94 × 10-11J
C) 1.53 × 10-11J
D) 2.94 × 10-11J
Correct Answer:
Verified
Q25: Which of the following statements is true
Q26: In electron capture, a nucleus captures an
Q27: The decay series starting with uranium-238involves a
Q28: Which of the following statements is true
Q29: In the context of the ionizing and
Q31: The SI unit of nuclear activity is
Q32: In the context of the ionizing and
Q33: The half-life of uranium-238is 4.5 billion years.
Q34: In the context of the nuclear binding
Q35: A tiny sample of a fabric is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents