Which of the following is the correct valence electron configuration of phosphorus?
A) 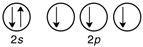
B) 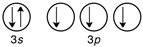
C) 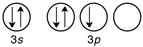
D) 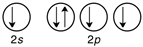
Correct Answer:
Verified
Q49: Which of the following is a representation
Q50: Which of the following metals (M)would produce
Q51: _ explains why orbitals can contain a
Q52: _ states that, electrons will not join
Q53: Which of the following violates the Pauli
Q55: Unlike the original attempt by Mendeleev, the
Q56: The element chlorine is found in the
Q57: Which pair of the following represents the
Q58: Which of the following is considered a
Q59: The highest-energy shell of an element that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents