Which of the statements is correct when referring to the following reaction? H2(g) + I2(g)  2HI(g)
2HI(g)
A) This reaction is reversible because the amount of heat given off by the forward reaction is the same as the heat given off by the reverse reaction.
B) The physical state of the reaction is found by using 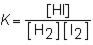 .
.
C) Most of the matter is on the right side of the equation.
D) There isn't a correct response provided.
Correct Answer:
Verified
Q28: Equilibrium: The following question(s)refer to the equilibrium
Q29: BrCl is put into an empty 1.00
Q30: Equilibrium: The following question(s)refer to the equilibrium
Q31: CFC's such as CF2Cl2, are associated with
Q32: Which statement is a correct application of
Q34: CFC's are in the environment because they
Q35: Which of the following statements about a
Q36: A catalyzed, aqueous chemical reaction occurs. Which
Q37: Equilibrium: The following question(s)refer to the equilibrium
Q38: Which statement is incorrect with reference to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents