Which of the following would be the equilibrium expression for the system given below? N2(g) + 3F2(g)  2NF3(g)
2NF3(g)
A) 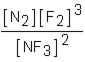
B) 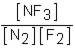
C) 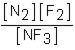
D) 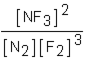
Correct Answer:
Verified
Q52: Ice has a(n)_ entropy than that of
Q53: An elderly person comes to you on
Q54: Based on the following equilibrium constants, which
Q55: A process that gains or accepts energy
Q56: A room filled with a 2:1 mixture
Q58: For the following energy diagram, which letter
Q59: A patient complains about having painful muscle
Q60: Which of the following is the proper
Q61: A reaction rate can be described using
Q62: Catalysts may lower the activation energy.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents