Phosphoric acid, H3PO4, undergoes three dissociation reactions. Which of the three acids is the weakest?
A) 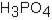
B) 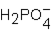
C) 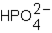
D) More than one response is correct.
Correct Answer:
Verified
Q18: What are the missing products in the
Q19: Identify the Brønsted acid(s)in the reaction. HIO3(aq)+
Q20: Identify all Brønsted base(s)in the reaction. N3-
Q21: How many equivalents are contained in 0.25
Q22: Which salt shifts the pH when dissolved
Q24: Antacids contain a substance that neutralizes hydrochloric
Q25: A 25.00 mL sample of H2SO4 acid
Q26: Identify the substance with the lowest pH.
A)orange
Q27: Which of the following is a weak
Q28: Which of the following mixtures would represent
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents