The pH of blood must remain stable between 7.35 and 7.45. One of the blood components is the bicarbonate ion, 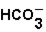 . Bicarbonate takes part in two reactions that are:
. Bicarbonate takes part in two reactions that are:
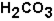 (aq)
(aq) 
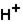 (aq)+
(aq)+ 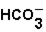 (aq)
(aq) 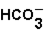

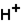 (aq)+
(aq)+ 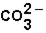 (aq)
(aq)
This set of equations indicates that this system is a good candidate to act as a buffer system used to hold the pH of blood reasonably stable.
Correct Answer:
Verified
Q82: The _ _ is a tabular
Q83: Water is able to act not only
Q84: Citrus fruits are high in both citric
Q85: Identify a true statement of acids.
A)Acids
Q86: List the rules to be followed
Q87: Gastroesophageal reflux disease (GERD)is the result of
Q88: The goals of an acid\base titration is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents