During a metabolic pathway the following reaction produced 3.00 moles of product. If Δ G°' for the hydrolysis of ATP is -7.3 kcal\mol, how much energy would be involved? 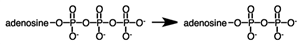
A) 7.3 kcal released
B) 7.3 kcal absorbed
C) 22 kcal absorbed
D) 22 kcal released
Correct Answer:
Verified
Q54: What property would lead scientists to believe
Q55: On which of the following vitamins can
Q56: Which of the following is not considered
Q57: The vitamin B1 (thiamin)deficiency disease is _
Q58: What would be the products of the
Q60: A patient comes to you suffering from
Q61: Several micronutrients are components of enzymes.
Q62: The reactive site in NADH is the
Q63: A function of ATP is to provide
Q64: Lipids are a source of energy and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents