Which of these would be expected to have a trigonal planar electron geometry?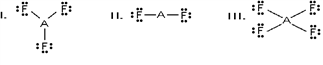
A) I and II
B) II and III
C) I, II, and III
D) I and III
E) I only
Correct Answer:
Verified
Q41: Which of these is the correct molecular
Q50: Which of these molecules will have a
Q53: Electronegativity is:
A)the ability of an atom to
Q54: Which of these theories is utilized in
Q55: Which of these is the correct molecular
Q56: Which of these molecules will have a
Q56: Which of the following results in bent
Q57: Which of these is the correct molecular
Q57: A molecule with tetrahedral electron geometry could
Q59: Which of these is the correct molecular
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents