Which particle completes this equation?
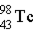 → ____ +
→ ____ + 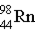
A) Alpha particle
B) Beta particle
C) Gamma particle
D) A positron
E) Proton
Correct Answer:
Verified
Q12: Uranium-238 decays by emission of an alpha
Q13: Which of these is the correct representation
Q14: Which type of radioactive emission has the
Q15: Which process best describes beta decay?
A)A proton
Q16: Which of these scientists is responsible for
Q18: Which type of radioactive emission has the
Q19: Which particle completes this equation? Q20: Krypton-85 decays by emission of a beta Q21: What was the secret code name for Q22: The half-life of phosphorous-33 is 25.3 days.
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents