Identify the element X present in the fourth row of the periodic table that forms the following ion:
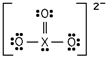
A) Ge
B) As
C) Se
D) Kr
E) Br
Correct Answer:
Verified
Q31: How many bonding pairs of electrons are
Q32: How many bonding pairs of electrons are
Q33: Which of these molecules will have a
Q34: The molecular geometry of a two-atom molecule
Q35: Identify the element X present in the
Q37: How many lone pairs of electrons are
Q38: How many lone pairs of electrons are
Q39: Which of these molecules contains two double
Q40: How many lone pairs of electrons are
Q41: Which of these is the correct molecular
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents