What is 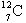 ?
?
A) There is no such thing.
B) an isotope of carbon with 7 neutrons and 12 protons
C) an isotope of carbon with 7 neutrons and 5 protons
D) an isotope of carbon with 5 neutrons and 7 protons
Correct Answer:
Verified
Q42: After three half-lives,3/4 of the nuclei in
Q44: An alpha particle is like 
Q50: The energy of nuclear radiation as it
Q51: Two isotopes have the same
A)mass number.
B)neutron number.
C)atomic
Q52: Einstein's equation E = mc2 states that
Q55: What does the 235 represent in uranium-235?
A)the
Q56: The expression(s)indicating the atomic number of a
Q58: How can an electron be ejected from
Q59: Low levels of ionizing radiation
A)are dangerous to
Q60: If someone tells you they have a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents