Exhibit 1-1 Consider the following figure that is a blow-up view of a region of a 50 mL burette to answer the following question(s) . 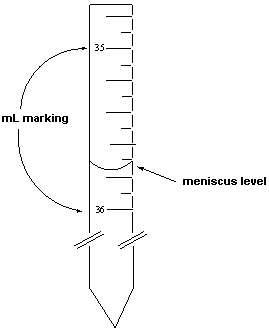 The density of ethanol was measured in two steps. The first step involved measuring its volume using a burette. The mass of this volume was then measured.
The density of ethanol was measured in two steps. The first step involved measuring its volume using a burette. The mass of this volume was then measured.
-Refer to Exhibit 1-1. If the mass of the ethanol delivered was 28.217 grams, what is the density of this liquid reported to the correct number of significant digits?
A) 0.7893 g/mL
B) 0.81 g/mL
C) 0.790 g/mL
D) 1.267 g/mL
E) 0.7784 g/mL
Correct Answer:
Verified
Q81: Liquid Helium boils at 4 K. What
Q82: A comfortable temperature for bath water is
Q83: Convert 26 ° C to kelvins.
A) 288
Q84: Convert 38 ° F to the Celsius
Q85: Exhibit 1-1 Consider the following figure that
Q87: The temperature of liquid nitrogen is 77
Q88: What is the volume of 25.0 g
Q89: What mass of platinum occupies a volume
Q90: What is the mass of 25.0 mL
Q91: What volume of gold has a mass
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents