Choose the correct net ionic equation for the reaction of HCl and NaOH taking place in H2O.
A) 2 H+ (aq) + OH - (aq) → H3O+ (aq)
B) H + (aq) + OH - (aq) H₂ O ( 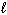 )
)
C) 2 Na+ (aq) + 2 Cl - (aq) → 2 NaCl (aq)
D) 2 H + (aq) + 2 Cl - (aq) + 2 Na + (aq) + 2 OH - (aq) 2 H₂ O ( 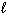 ) + 2 NaCl (aq)
) + 2 NaCl (aq)
E) none of these
Correct Answer:
Verified
Q2: Which compound listed below is not soluble
Q3: Which of the following ionic compounds are
Q4: Choose the correct net ionic equation for
Q5: Exhibit 4-1 Consider the precipitation reaction below
Q6: A chemist places AgNO3 in one flask
Q8: Which compound listed below is soluble in
Q9: What products are expected and what are
Q10: Which compound listed below is not soluble
Q11: Which compound listed below is soluble in
Q12: Exhibit 4-1 Consider the precipitation reaction below
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents