Given the equation below, what is D H for burning 8.0 g of C₂ H₂ (g) in excess O₂(g) ?
2 C₂ H₂ (g) + 5 O₂(g) 4 CO₂ (g) + 2 H₂ O ( 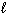 ) D H = - 2600 kJ
) D H = - 2600 kJ
A) - 200 kJ
B) - 400 kJ
C) - 800 kJ
D) - 1600 kJ
E) none of these
Correct Answer:
Verified
Q10: Energy possessed by a sample of matter
Q11: Which of the following reactions releases heat
Q12: Given the three statements below, which answer
Q13: Given the equation below, what is D
Q14: Given the thermochemical equation:
2 SO2 (g) +
Q16: Exhibit 5-1 Consider the reaction of Magnesium
Q17: Which of the following reactions absorbs heat
Q18: Exhibit 5-1 Consider the reaction of Magnesium
Q19: Given the equation below, what is D
Q20: Given the three statements below, which answer
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents