Liquid hydrogen peroxide is an oxidizing agent in many rocket fuel mixtures because it releases oxygen gas on decomposition as shown below. 2 H₂ O₂ ( 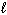 ) 2 H₂ O (
) 2 H₂ O ( 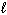 ) + O₂(g) D H rxn = - 196 kJ How much heat is released when 25.0 grams of H₂ O₂ decomposes?
) + O₂(g) D H rxn = - 196 kJ How much heat is released when 25.0 grams of H₂ O₂ decomposes?
Molar Mass(H₂ O₂) = 34.01 g/mol
A) - 533 kJ
B) - 288 kJ
C) - 144 kJ
D) - 133 kJ
E) - 72.0 kJ
Correct Answer:
Verified
Q3: Given the equation below, what is D
Q4: Consider the following reaction and its corresponding
Q5: Given the equation below, what is D
Q6: What mass of propane gas (C3 H8
Q7: What mass of nitrogen must burn in
Q9: Given the following reaction and its corresponding
Q10: Energy possessed by a sample of matter
Q11: Which of the following reactions releases heat
Q12: Given the three statements below, which answer
Q13: Given the equation below, what is D
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents