What is the standard molar enthalpy change D H rxn for the following reactions using the standard molar enthalpies of formations provided?
SiCl 4 ( 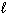 ) + 2 H₂ O (
) + 2 H₂ O ( 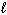 ) SiO₂ (s) + 4 HCl (g) D H rxn = ???
) SiO₂ (s) + 4 HCl (g) D H rxn = ???
D H f (SiCl 4 ) = - 640.1 kJ/mol D H f (SiO₂ ) = - 910.9 kJ/mol D H f (H₂ O) = - 285.8 kJ/mol D H f (HCl) = - 92.3 kJ/mol
A) - 2491.8 kJ/mol
B) - 1929.1 kJ/mol
C) - 77.3 kJ/mol
D) - 68.4 kJ/mol
E) None of these
Correct Answer:
Verified
Q68: Given the following Thermodynamic data:
D H f
Q69: Given the data below, what is D
Q70: For which of the equations shown below
Q71: What is the enthalpy of reaction D
Q72: Which of the following defines the heat
Q74: Given the following experimental data:
D H rxn
Q75: Given the data below, what is D
Q76: Given the data below, what is D
Q77: Given the data below, what is D
Q78: Which of the equations listed below corresponds
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents