Three sets of quantum numbers are listed below. Pick the best answer.
I. n = 2, 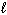 = 2,
= 2, 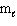 = 2, m s = 1 / 2
= 2, m s = 1 / 2
II. n = 4, 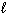 = 0,
= 0, 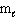 = 0, m s = 1 / 2
= 0, m s = 1 / 2
III. n = 3, 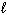 = 2,
= 2, 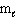 = 3, m s = 1 / 2
= 3, m s = 1 / 2
A) I and II are allowed sets, III is not
B) II and III are allowed sets, I is not
C) all 3 are allowed sets
D) only II is an allowed set
E) all 3 are not allowed sets
Correct Answer:
Verified
Q26: According to the Bohr model for a
Q27: What quantum number(s) provide(s) information with respect
Q28: What quantum number(s) provide(s) information with respect
Q29: What quantum number(s) is(are) required to completely
Q30: The energy of the electron in the
Q32: What quantum number(s) is(are) required to completely
Q33: The quantum number l for an electron
Q34: Calculate the wavelength of light emitted when
Q35: From the restrictions on the three quantum
Q36: Given the three statements below, pick the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents