A subshell is described by assigning values to the quantum numbers
A) n only
B) n, 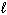 , and
, and 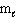
C) n and 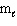
D) n and 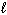
E) 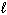 only
only
Correct Answer:
Verified
Q51: Exhibit 7-1 Consider the shapes listed below
Q52: How many possible orientations are allowed when
Q53: How many electrons can be placed in
Q54: Which full set of four quantum numbers
Q55: The possible values of the magnetic quantum
Q57: What is the order for filling the
Q58: What is the order for filling the
Q59: The possible values of the magnetic quantum
Q60: What is the maximum number of electrons
Q61: Which orbital diagram for the ground state
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents