Which full set of four quantum numbers would be expected for the highest energy electron for a potassium atom?
A) n = 4, 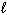 = 0,
= 0, 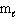 = 0, m s = +1/2
= 0, m s = +1/2
B) n = 4, 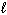 = 1,
= 1, 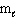 = 1, m s = +1/2
= 1, m s = +1/2
C) n = 4, 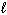 = 2,
= 2,  = 2, m s = +1/2
= 2, m s = +1/2
D) n = 3, 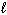 = 1,
= 1,  = 1, m s = +1/2
= 1, m s = +1/2
E) n = 3, 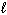 = 2,
= 2, 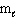 = 2, m s = +1/2
= 2, m s = +1/2
Correct Answer:
Verified
Q49: How many electrons can be placed in
Q50: What are the possible Q51: Exhibit 7-1 Consider the shapes listed below Q52: How many possible orientations are allowed when Q53: How many electrons can be placed in Q55: The possible values of the magnetic quantum Q56: A subshell is described by assigning values Q57: What is the order for filling the Q58: What is the order for filling the Q59: The possible values of the magnetic quantum![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents