The highest energy electron for Ga (Z = 31) could be designated by which of the following four quantum numbers (only one correct possibility is shown, others are possible) ?
A) n = 3, 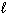 = 1,
= 1, 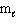 = 1, m s = 1/2
= 1, m s = 1/2
B) n = 4, 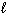 = 1,
= 1, 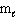 = - 1, m s = 1/2
= - 1, m s = 1/2
C) n = 3, 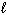 = 0,
= 0, 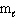 = 0, m s = 1/2
= 0, m s = 1/2
D) n = 4, 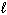 = 2,
= 2, 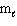 = 1, m s = 1/2
= 1, m s = 1/2
E) n = 5, 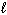 = 2,
= 2, 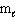 = 1, m s = 1/2
= 1, m s = 1/2
Correct Answer:
Verified
Q14: The ground state electron configuration of Co
Q15: How many electrons would be in the
Q16: The ground state electron configuration of Se
Q17: Which of the following is an acceptable
Q18: The valence shell orbital configuration of the
Q20: The ground state electron configuration of Co3+
Q21: How many unpaired electrons does the ground
Q22: The correct ground state electron configuration for
Q23: How many valence electrons does a silicon
Q24: How many unpaired electrons does the ground
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents