Given the three statements below, pick the best answer.
I. The Lewis symbol of boron is 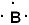
II. KCl should have a greater lattice energy than BeO.
III. The Lewis symbol Mg is correct for a magnesium atom.
A) all three are true
B) I and II are true, III is false
C) I and III are true, II is false
D) II and III are true, I is false
E) only III is true
Correct Answer:
Verified
Q14: Which atom from the list below normally
Q15: Which compound listed below should have the
Q16: Taking into account the size of ions
Q17: A chemical property of magnesium metal, Mg
Q18: Which of the following is a false
Q20: Which species listed below has the largest
Q21: The Lewis structure for formaldehyde (CH2O) is:
A)
Q22: The molecule H2NCH3 has:
A) 6 bonding pairs
Q23: How many electrons (both lone and bond
Q24: Which Lewis structure below obeys the octet
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents