Which Lewis structure below obeys the octet rule for every atom in the structure of COCl2 and uses the proper number of valence electrons?
A) 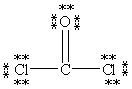
B) 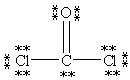
C) 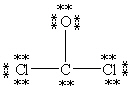
D) 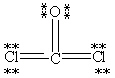
E) 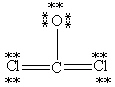
Correct Answer:
Verified
Q49: Which Lewis structure below obeys the octet
Q50: How many available valence electrons are used
Q51: Consider the polyatomic ion, CN1 - .
Q52: Which Lewis structure below obeys the octet
Q53: Which Lewis structure below obeys the octet
Q55: Which Lewis structure below for hydrazine, N2H4,
Q56: Which Lewis structure below obeys the octet
Q57: Consider the polyatomic ion, ClO1 - .
Q58: Consider the polyatomic ion, NO1+. Which Lewis
Q59: Consider the polyatomic ion, OH1 - .
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents