Based upon general trends in electronegativity, which polar covalent chemical bond(s) listed below is(are) properly labeled using the vector convention discussed in class?
(Recall that the vector points toward the most electronegative atom in the bond.)
I. 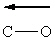
II. 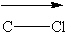
III. 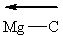
A) II only
B) I and II
C) I and III
D) II and III
E) All of these
Correct Answer:
Verified
Q60: Which Lewis structure below obeys the octet
Q61: Arrange the following bonds in order of
Q62: Which bond listed below is most polar?
A)
Q63: Which of the following bonds is most
Q64: Which of the following bonds has the
Q66: Consider the polyatomic ion, NO21 - .
Q67: Consider the polyatomic ion, (SO3)2 - .
Q68: Which bond would be most polar?
A) O
Q69: Which of the following elements has the
Q70: Which of the following bonds would be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents