A possible Lewis structure for BeCl₂ is given below. 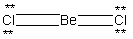 Why does this resonance form contribute very little to the true electronic structure for this molecule?
Why does this resonance form contribute very little to the true electronic structure for this molecule?
A) It does not use the proper number of valence electrons.
B) It does not obey the octet rule for Beryllium.
C) It does not obey the octet rule for Chlorine.
D) The formal charge of the Beryllium atom is +2 and the formal charge of each Chlorine atom is - 1. This provides a charge separated compound.
E) The formal charge of the Beryllium atom is - 2 and the formal charge of each Chlorine atom is +1. This places a negative charge on a metal and a positive charge on a highly electronegative non-metal atom.
Correct Answer:
Verified
Q94: Three possibly correct resonance forms of N₂O4
Q95: A Lewis structure for OF 2 ,
Q96: Based upon a formal charge analysis, what
Q97: Pick the correct Lewis structure shown below.
A)
Q98: The difference in electronegativity between a boron
Q100: Possible resonance forms for ClO3 - are
Q101: Given the average bond energies listed below,
Q102: What is the correct Lewis structure for
Q103: Which molecule listed below has a total
Q104: Given the average bond energies listed below,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents